UK is testbed for mass vaccination with gene therapies, leading to global rollout
DEFEAT CAPITALISM AND ITS DEADLY SPAWN, IMPERIALISM
ecological murder •
Editor's Note: No doubt that Hedley Rees is a valuable witness against the global pharma monster, and, for that, we should be grateful. However, in reading his exposés and diverse complaints, it is always necessary to bear in mind that his thinking remains largely and narrowly bound by the capitalist way of looking at things, hence his solutions and proposals always emerge, tortuously, from the maze of liberaloid fixes permitted by the capitalist playbook. In terms of logic, this is like going in circles. It may not occur to Rees, or it may be ideologically unpalatable, to contemplate that most if not all of the issues he denounces so passionately would swiftly vanish under a regime in which the profit imperative was completely removed from the center of healthcare. —PG
Originally Nov 18, 2022
Get the inside track here...
The first clue
The first clue was confirmed when I had an email from Jonathan Gilthorpe about a Swedish investigative reporter wanting to interview me on GMP. Jonathan is fully awake and we exchange occasional emails as things pop out the woodwork.
I’d been concluding, by dot joining, that the UK was the epicentre of the SARS-CoV2 injections rollout, but the description ‘testbed’ brought instant clarity.
Of course, if they could get away with it in one country with a very ‘friendly’ Regulatory Authority, it would then be EMA, FDA and all around the rest of the world…and here, in the UK, we have the extremely friendly, even ‘enabling’ Regulatory Authority, MHRA.
We spoke about this a few days ago, as below:
The crux of this is that MHRA changed its Regulations to allow gene therapies, and other advanced medicinal products, to be manufactured without the safety umbrella of there being a proper Quality Management System in place. Dangerous stuff!!!
It takes me back to 2013
For reasons I can’t explain, my mind flashed back to 2013, when I first worked in the gene therapy arena, under the umbrella of my company PharmaFlow.
Then, I consulted to a contract development and manufacturing organisation (CDMO) for gene therapies, named Oxford Biomedica (OXB). The job was on a UK Government funding call, titled the Advanced Manufacturing Supply Chain Initiative (AMSCI) Round 3.
The feedback UKs Office for Life Sciences received was that in the two previous rounds of AMSCI, no life sciences companies were successful. The reason given was they were using a scientific mindset for a challenge that required an engineering and production systems approach. The Office for Life Sciences asked if I could find a company to submit a bid, and steer them through to a successful conclusion.
To cut a long tale short – I found a company and the bid was successful: Oxford BioMedica Wins Significant Funding via a Competitive Award from UK Government’s Advanced Manufacturing Supply Chain Initiative.
You won’t find PharmaFlow on the announcement, even though I had personally recruited Cranfield University and Heart of England NHS Foundation Trust!
That’s often the way I suppose. I felt betrayed at the time, and even more so when they dropped me from the project and fired the Director of Manufacturing who I had been working closely with. That’s business, as they say!
The important bit
Anyway, this is the important bit. That was the last contact I had with the company, but UK Government stayed very much engaged, as I’ve now realised:
Chief Secretary to the Treasury visits Oxford BioMedica
The Office for Life Sciences were hugely impressed by the potential for gene therapies manufactured by OXB in the UK, especially when OXB signed a £100 million agreement with Novartis to supply the viral vector for Kymriah (which they were developing while I was there):
Kymriah approved - royalties to OXB assured
OXB then went on to develop and manufacture the adenovirus vector (Kymriah is a lentiviral vector, very similar) for AstraZeneca:
https://www.oxb.com/news-media/press-release/oxford-biomedica-signs-supply-agreement-astrazeneca-expand-manufacturing
It begins: “Kymriah (tisagenlecleucel/CTL019), for which Oxford BioMedica (OXB) provides the key lentiviral vector component, has been approved by the FDA for the treatment of patients up to 25 years of age with refractory/relapsed (second or later relapse) B-cell acute lymphoblastic leukaemia (ALL). Novartis has announced that the price for the one-off treatment will be $475k (c £370k), an increase over our previous assumption of £300k”
Kymriah has significant side effects of Cytokine Release Syndrome and neurological toxicities, which appear (to the unqualified me) as similar to the AZ side-effects being reported.
Why am I saying all this?
…because the evidence that the UK IS the testbed for gene therapy-based products (especially mRNA) is extremely persuasive.
ADDENDUM
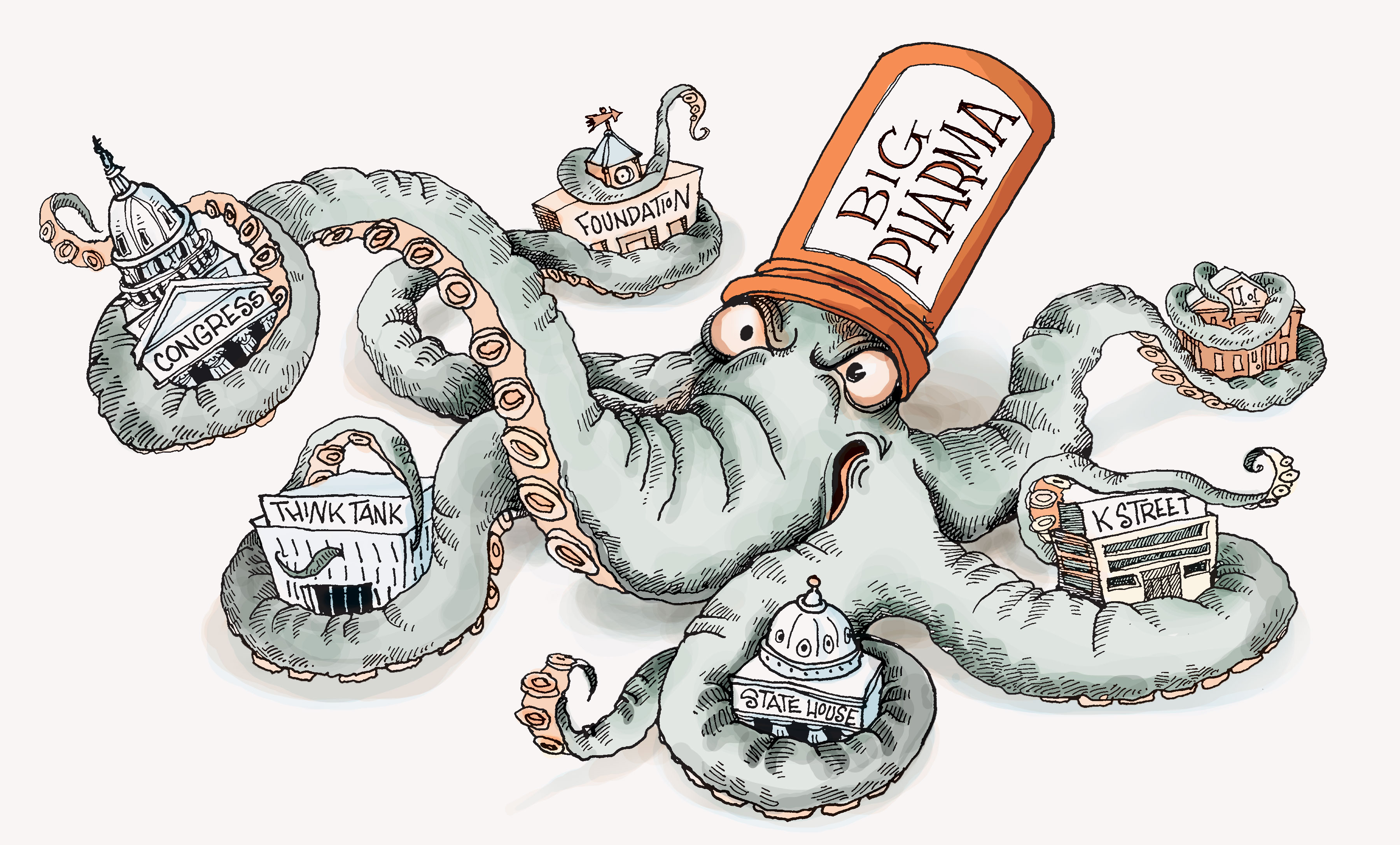
Taming the pharma monster with Hedley Rees
With the aim of re-aligning the industry away from patents and towards patients, Hedley Rees, veteran of the UK pharma sector and now managing consultant at PharmaFlow, spoke to us about the best way to tame and reform the ‘monster.’

To correct this and approach modernising the industry, Rees has written a booked titled ‘Taming the Pharma Monster’, and has used his consultancy PharmaFlow, as well as Welsh-led change initiative Friends of Medicines Modernisation to organise a ‘Medicines for the 21st century’ conference.
Rees and his partners are in the middle of composing a White Paper based on the conference’s conclusions, which will be sent to the House of Commons Health and Social Care Committee, as well as organising a non-profit company to support patients and patient advocacy groups in challenging the industry.
Allie Nawrat: Why did you describe the pharma industry as ‘a monster’ in the title of your new book?
Hedley Rees: I think there is now a perception that as a system it is similar to the banking system during the 2008 financial crash, and that it is certainly not beneficial to patients or healthcare professionals.
If you take the high profile example of insulin in the US, there are three companies in the US who are charging astronomically high prices for insulin. Patients in the US are dying because they have started to ration their insulin or are not taking it at all.
The core issue is that [companies have] patents [,which] create monopolies or oligopolies, so when drugs eventually get to market, pharma companies can basically choose their price based on the [maximum] they think the market will pay.
AN: When did this situation emerge and how has it consolidated itself?
HR: We have to go back 40, 50 years to when the industry was vertically integrated – the large pharmaceutical companies owned the facilities for making drugs, they employed the people and they sourced from suppliers in a vertically integrated way. Companies were able to get patents for the process, not just the molecules.
Therefore, it looked as if blockbuster revenues came from having a patented drug and [employing] clever sales and marketing, and so the rest of the industry copied that approach.
We are writing a white paper to send to the Health and Social Care Committee, in which we are going to ask really searching questions, such as why is the industry allowed to test its own products – there is no other industry that does that – and why aren’t there post-mortems on failed clinical trials?
There has to be learning in there about what went wrong and what didn’t – but all that is kept secret by the companies. If you compare it to the aviation industry, when there is a plane crash all the information is shared immediately across the industry as to what went wrong and what is being done to put it right.
You have to ask yourself, why doesn’t that happen in pharma? It is because we feel like medicines are different to other sectors, but they are not, there should be an obligation on those companies [like there is in other industries] to investigate failures and explain why they happened.
AN: What can be done to break this negative cycle and bring the industry into the modern era?
HR: I am repeating what people at the coalface – researchers, scientists and physicians – are saying to me. We want the industry to provide more evidence that their molecule could get to market, before the patent was awarded. Unless you take away the incentivisation of the patent, nothing is ever going to change.
There needs to be a consultation of trade bodies, like the BioIndustry Association and the Association of the British Pharmaceutical Industry, facilitated by government, which actually asks these questions around patents. It is about going back 40 years, looking at the benefits of vertical integration and moving back to collaboration.
AN: Technology is often viewed as a silver bullet. What role do you think emerging technologies can play?
HR: We need to incentivise pharma companies to spend more time at the early stage to invest in predictive technologies, such as in vitro, ex vivo, Insilco technologies and organ on a chip, that really have moved on a long way, but that the industry has not embraced.
There is a lot of hype about AI because the people who have got the technology want to sell it. AI is an enabler, but if you have got a system that is overly complex, clunky, and not properly integrated then AI or machine learning will not doing anything. It has to be supported for the people at the coalface.
Print this article
Unfortunately, most people take this site for granted.
DONATIONS HAVE ALMOST DRIED UP…
PLEASE send what you can today!
JUST USE THE BUTTON BELOW
[/su_spoiler]
![]() Don’t forget to sign up for our FREE bulletin. Get The Greanville Post in your mailbox every few days.
Don’t forget to sign up for our FREE bulletin. Get The Greanville Post in your mailbox every few days.
[newsletter_form]
[premium_newsticker id=”211406″]

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License